Your trusted partner for a comprehensive Clinical Event Committee solution
Partners you can trust
to manage your program
Deep clinical domain
expertise when it matters most
Enhance your data quality
with our comprehensive web-based platform
White paper
Biotech and Pharma Execs Weigh in on the Role of Clinical Adjudication in Clinical Trials
Partners you can trust to manage your program
Collaborative project plans are critical success factors in effectively meeting timelines and deliverables. Our team of program and project managers has over 100 years of cumulative experience in clinical trial adjudication. We partner with you from day one and support your program through the end of the study.
- Initiate rapid launch of new studies or transition of existing data with rescue studies
- Engage professional project managers, nurses and medical writers to ensure your study is consistent with charter requirements
- Our project management team fully manages the coordination of Clinical Event Committee (CEC) meetings
- Facilitate global trials at the country level with dedicated project managers, localized language support and translated user interface and training materials — no on-premise servers required

Deep clinical domain expertise when it matters most
Our vast global network of medical experts specializes in specific clinical trial endpoint therapeutic areas, including (but not limited to) pulmonology, cardiovascular, gastroenterology, MSK, and neuroscience.
- Expert guidance on charter design by our veteran adjudication specialists and clinicians
- Draw from Clario’s 30 years of medical imaging expertise and extensive network of key opinion leaders across nearly all therapeutic areas
- Gather insights throughout your trial with full data transparency that displays research site, user and adjudicator performance metrics visually
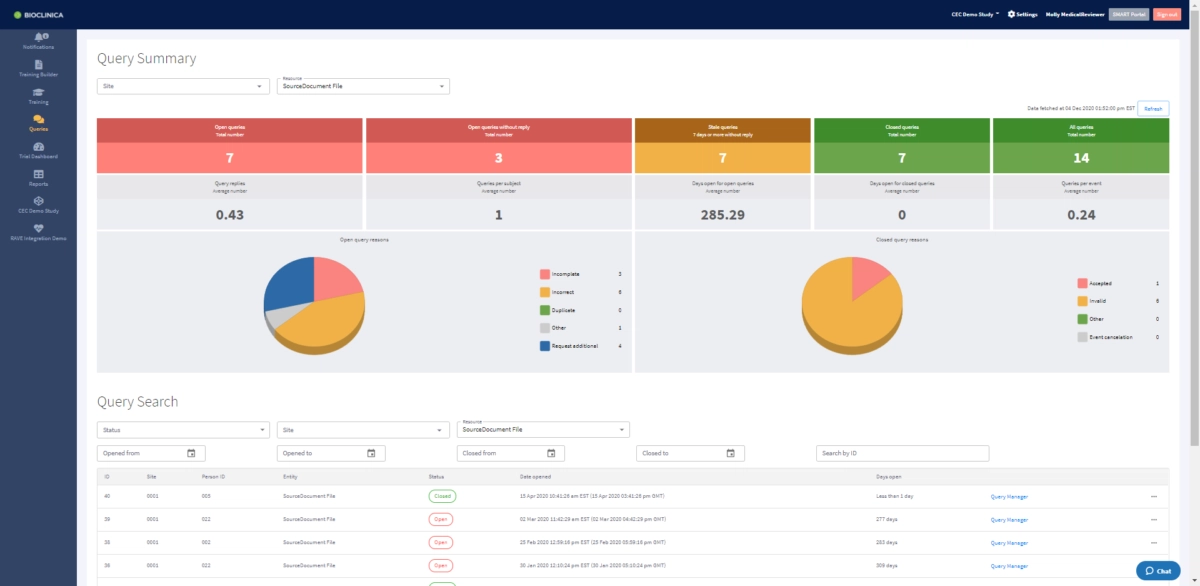
Enhance your data quality with our comprehensive web-based platform
A centralized online repository of source documents, DICOM, videos, photos, and ECGs, simplifies the management of the clinical trial adjudication process.
- Achieve 100% de-identification of all source documents, images, videos and photos with our artificial intelligence (AI) technology, as well as quality control (QC) and query management for all source document types for patient privacy
- Enforce compliance with 21 CFR Part 11 and EU GDPR requirements
- Reduce manual errors and costs with automatic routing and de-identification
- Integrate easily with 3rd-party applications, including Electronic Data Capture (EDC) and safety systems
- Address questions about clinical trial adjudication quickly with access to live support 24/7/365 via chat, phone or email within the platform
Talk to a specialist
Our team of experts is available to address questions you may have. Submit your contact information and we’ll be in touch shortly.