Clario’s Image Workflow adds a layer of structure and visibility to optimize your clinical trial image review process. Our secure, cloud-based medical image analysis software platform enables a centralized approach to manage your datasets. Create custom user workflows for image viewing, streamlining processes and automating reports. You’ll also be able to adhere to your trial’s stringent timeline with complete transparency into every step of the image review process to identify potential bottlenecks.
Create custom, automated workflows for clinical trial images to boost image review productivity
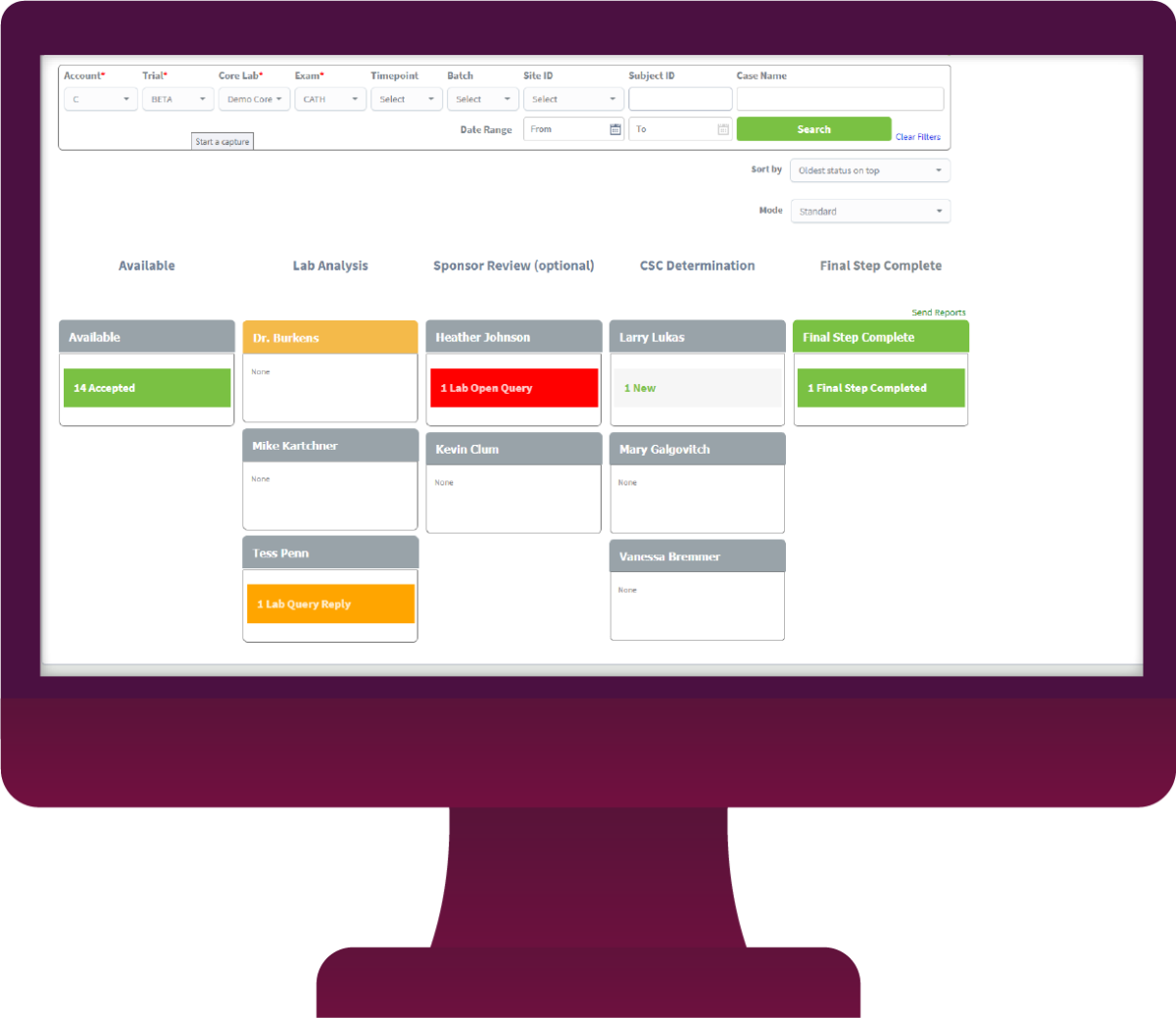
Track Your Image Review Process
WIth custom-designed workflows, you always know the status of the image analysis process. You can use Image Workflow’s electronic data capture (EDC) capabilities for image interpretation results as well.
Keep Stakeholders on Track
Restrict worklist access with tightly controlled user permissions that keep reviewers focused on their personal work queue.
Increase Efficiency
Reduce time wasted hunting for images with the ability to view images online, download them, or have them sent automatically to a reader’s workstation.
Protect Reviewer Safety
Keep the site – and reviewer identities – blinded to protect privacy with a full online query management system.
Simplify Reporting
Automatically create and email custom reports directly to designated recipients to keep project stakeholders updated on key milestones or emerging concerns.
View Performance Metrics
Track each step and user’s activity to ensure KPIs are being met.
Enforce compliance, reduce costs and enhance transparency with our medical image analysis software
- Enforce compliance: Comply with key data integrity and patient privacy regulations such as 21 CFR Part 11, EU GDPR and others.
- Reduce costs: Maximize precious research funds by automating the majority of the image coordinator workload, accomplishing more in less time.
- Avoid bottlenecks: Leverage complete process transparency to eliminate the “black box” phenomenon and avoid unexpected workflow issues that can endanger the trial’s timeline.
- Customer support you can count on: Our multilingual customer support team is available to assist you anywhere in the world with image uploads, transfers and viewing — 24/7/365. Our image quality control technicians, data managers and operations teams are dedicated to image management and to making your Image Workflow system work for you.
Advanced workflow optimization
- Enable customizable triggers to launch the image review process
- This medical image analysis software creates batches of cases that move through the workflow together
- Move and answer multiple cases at once
- Leverage blinded adjudication with automatic field comparison
- Divide cases into multiple copies or create sub-cases for sidebar analysis
Easily store and share
21 CFR Part 11 and EU GDPR-compliant images anywhere in the world from any device for use in future clinical trial projects or audits.
Talk to a specialist
Our team of experts is available to address questions you may have. Submit your contact information and we’ll be in touch shortly.